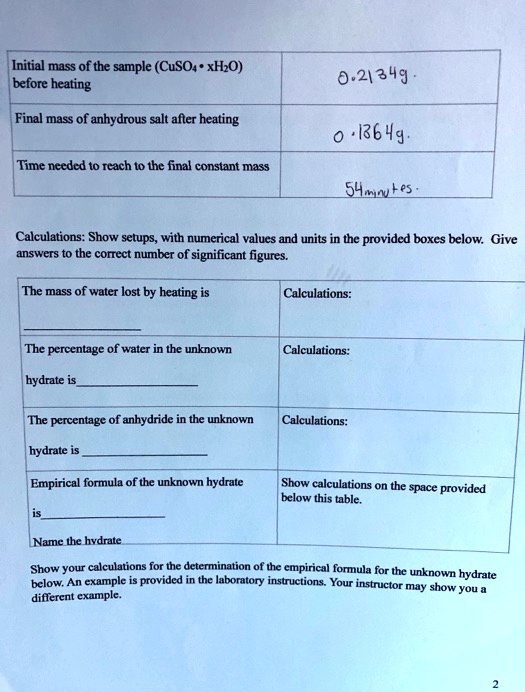
SOLVED: Initial mass of the sample (CuSO4 * xHO) before beating 0.21349 Final mass of anhydrous salt after heating Time needed t0 reach t0 the final constant mass S41uLes Calculations: Show setups;
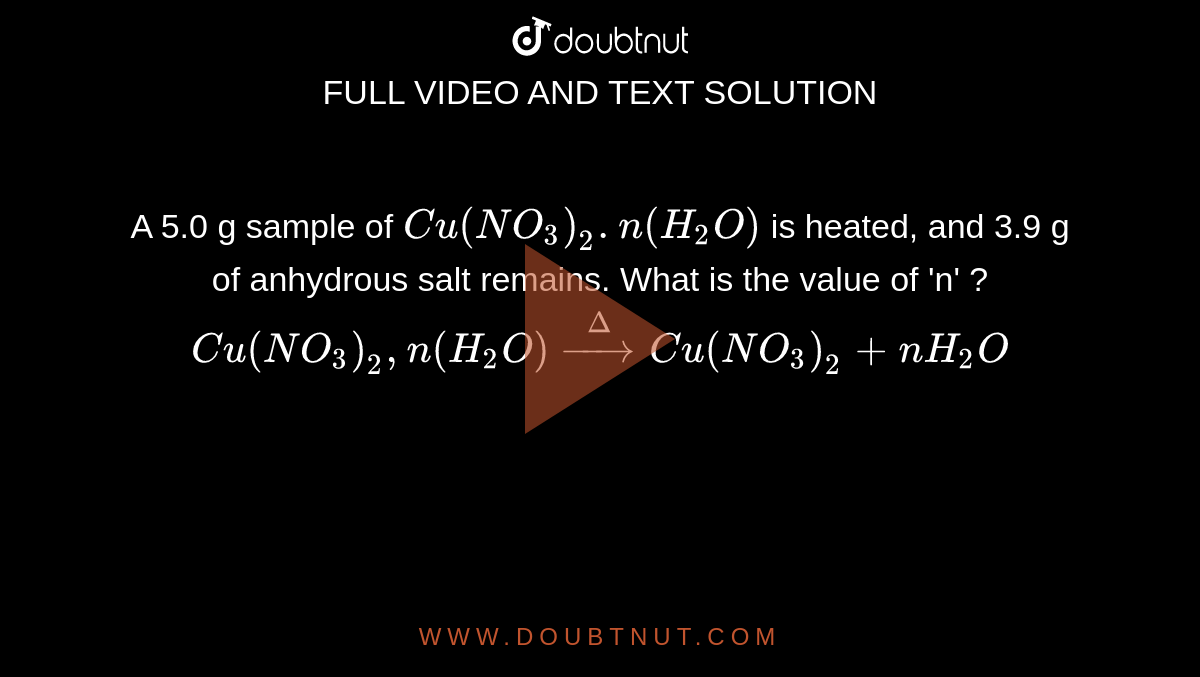
A 5.0 g sample of Cu (NO(3))(2).n (H(2)O) is heated, and 3.9 g of anhydrous salt remains. What is the value of 'n' ? Cu(NO(3))(2),n(H(2)O)overset(Delta)toCu(NO(3))(2)+nH(2)O

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol ^-1 of heat. The value of Δ H(hydration) of AB is - 29.4 J mol ^-1 . The

SOLVED: Formula (Molar) mass of anhydrous salt (on bottle) Number of grams of HzO per 100.0 g hydrate *49 14.8 Number of moles HzO per 100.0 g hydrate 1q.8 18.0 Da moles

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol^-1 of heat. The value of ΔHydration AB is - 29.4 J mol^-1 . The heat of dissolution of
3 anhydrous salt to be 208.3 g. Using Equation 2, determine the number of moles of the anhydrous salt. Equation 2 Number of mol
One mole of anhydrous salt AB dissolves inwater and liberates 15 J mol 1 of heat. The value ofdrs fon of AB is 20.5 J mol 1. Hence, the enthalpyof dissolution of



:max_bytes(150000):strip_icc()/anhydrous-chemistry-definition-603387_final-46e5c09532c045568b0a07dfddba7397.png)
.PNG)










